In adult patients with R/R B-cell precursor ALL
OS and RFS observed in the ~2-year analysis1,2
56% of patients were alive at the ~2-year analysis (OS rate KM estimate)2
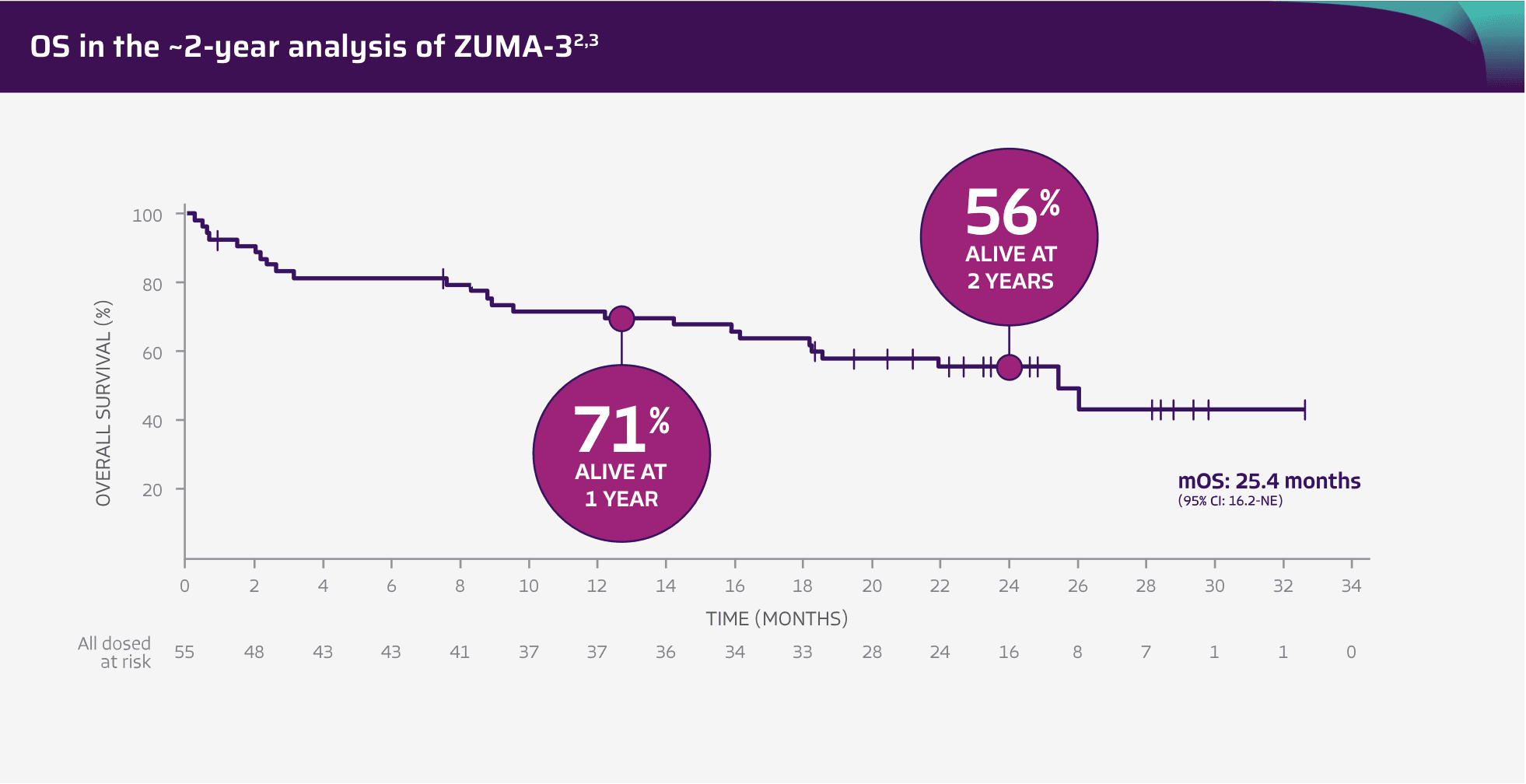
Adapted from Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study, J Hematol Oncol, 2022;15(1):170, © 2022 The Author(s), https://creativecommons.org/licenses/by/4.0. The copyright owner disclaims all representations and warranties regarding the licensed material.
Pinch to zoom
The tick marks represent censored patients. Subjects who have not died by the analysis data cut-off date will be censored at their last contact date.4
26.0 months mOS in patients with CR/CRi (95% CI: 21.9-NE)2
- The ZUMA-3 primary analysis publication reported that mOS was 18.2 months (95% CI: 15.9-NE) at a median study follow-up of 16.4 months2
- OS was a secondary endpoint of the ZUMA-3 phase 2, single-arm, open-label study and was not the primary objective of the study3
- The ~2-year OS analysis included patients who subsequently received allo-SCT (n=10) while in remission and who started new anti-cancer therapy (n=6). The KM estimate of OS at 2 years was 56% (n=55; 95% CI: 0.41-0.68). The KM mOS was 25.4 months (n=55; 95% CI: 16.2-NE)2
- OS data are not included in the USPI. OS data are descriptive and should be carefully interpreted in light of the single-arm design
15.5 months RFS in patients who achieved CR/CRi (n=39 responders)2
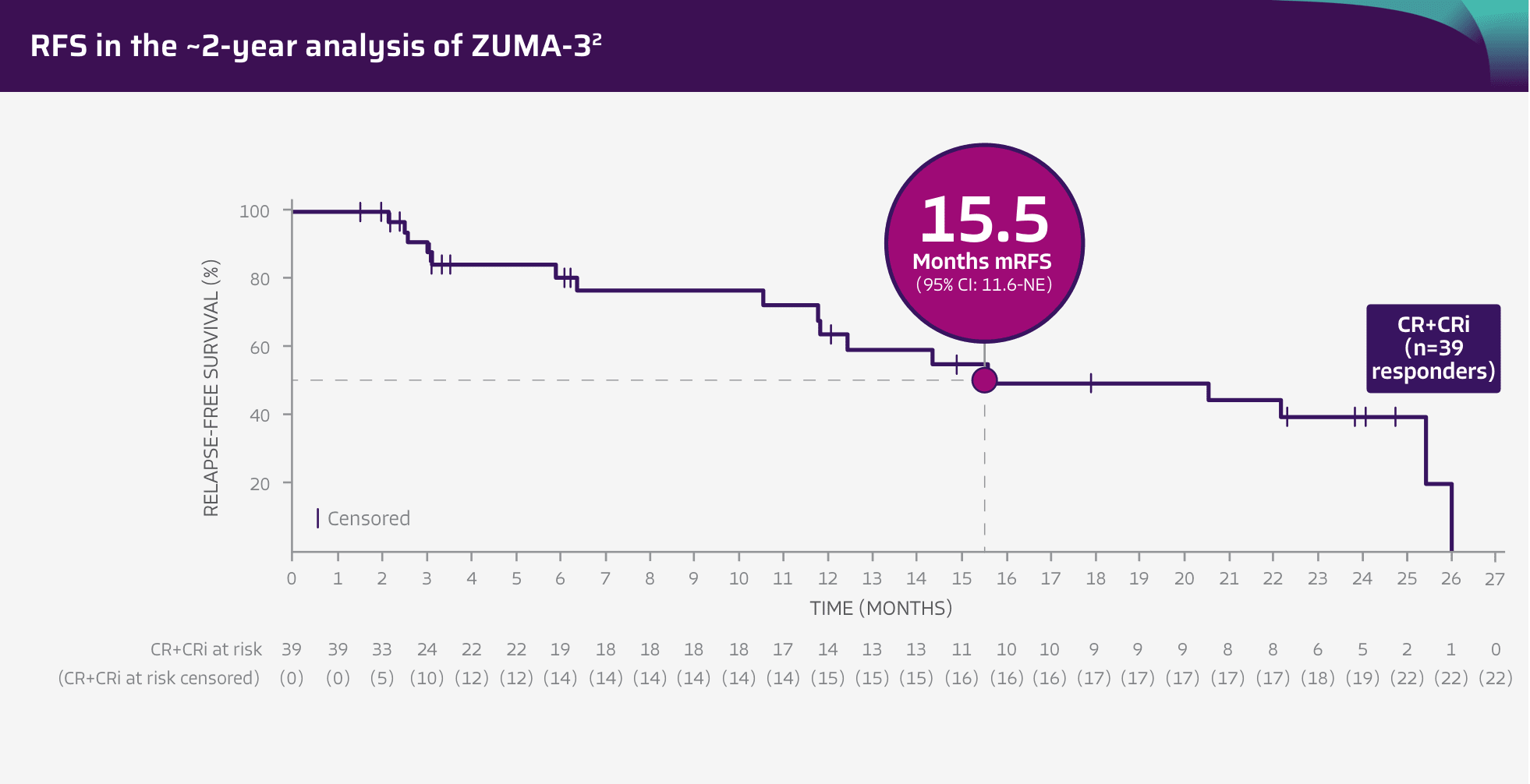
Adapted from Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study, J Hematol Oncol, 2022;15(1):170, © 2022 The Author(s), https://creativecommons.org/licenses/by/4.0. The copyright owner disclaims all representations and warranties regarding the licensed material.
Tick marks represent censored patients. Subjects not meeting the criteria for relapse by the analysis data cut-off date will be censored at their last evaluable disease assessment date.4
Pinch to zoom
- RFS was a secondary endpoint of the ZUMA-3 phase 2, single-arm, open-label study3
- RFS data are not included in the USPI. RFS data are descriptive and should be carefully interpreted in light of the single-arm study design
Brexucabtagene autoleucel (TECARTUS®) is recommended by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for adult patients with R/R B-cell ALL5:
- Listed as a preferred regimen option for patients with R/R Ph- B-cell ALL only (NCCN Category 2A)
- Listed as an other recommended regimen option for patients with R/R Ph+ B-cell ALL following therapy that has included TKIs (Category 2A)
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
ALL=acute lymphoblastic leukemia; allo-SCT=allogeneic stem cell transplant; CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; KM=Kaplan-Meier; mOS=median overall survival; mRFS=median relapse-free survival; NCCN=National Comprehensive Cancer Network; NE=not estimable; OS=overall survival; Ph=Philadelphia chromosome; RFS=relapse-free survival; R/R=relapsed or refractory; TKI=tyrosine kinase inhibitor; USPI=US Prescribing Information.
References: 1. TECARTUS® (brexucabtagene autoleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. 3. Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 4. Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502 (suppl). doi:10.1016/S0140-6736(21)01222-8 5. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed July 15, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.